0 - Why and how to make your own soap?
1 - How: the saponification process
The process of saponification requires a precise quantity of lye; this quantity varies depending on the oil(s) you use. So always use a lye calculator, even if you reuse a recipe seen on the internet (check twice!).
1 - How: the saponification process
The process of saponification requires a precise quantity of lye; this quantity varies depending on the oil(s) you use. So always use a lye calculator, even if you reuse a recipe seen on the internet (check twice!).
The Sage
I mostly use The Sage
calculator
- Make sure to choose the right weight measurement.
- Sodium hydroxide (already checked) is the lye we use for solid soap. Potassium hydroxide is used for liquid soap.
- If you're using lye flakes/beads, just fill in the oil information. In some countries, at least in France, you can buy water and lye already mixed, that's what "liquid lye solution" is for. The concentration that you have to write in the box must be around 30%.
***
- Enter the amount for each oil you want to use and then click on "Calculate lye"
***
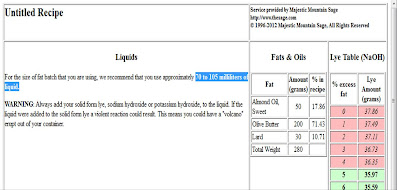
- The next page will give you information on the quantity of water (or another liquid) and caustic soda to use. The Sage gives a bracket of water amounts that can be used; to begin with, I recommend calculating and using the average. Here: between 70 and 105 ml ---> 86 ml or grams.
***
- The lye table gives you the quantity of soda you must use depending on the excess fat/superfat you want. The more superfat you want the soap to be, the less lye you will use. In other words, you will use a given amount of oil and make a lye reduction. It's just one way to do it (read more about superfatting here).
- A superfat between 5 % and 8 % is the most common and good enough for the skin. More than 8 %, the soap may not be hard enough and it will make your shower greasy. Less than 5 % is not especially recommended for the skin but for laundry soap, I choose 0 % or 1 % as a safety precaution.
*****
Soapcalc
I use also this other calculator
sometimes, just to check (you're never too safe with caustic soda).
- Again, NaOH (lye for solid soap) is already checked (1 on the picture), and you have to choose a weight measurement (2).
- The first difference between this calculator and The Sage is that you have to enter the total amount of oil you want to use (it's automatically 500 grams) (2). You choose your oils (5) by clicking on the drop-down menu. Then you fill in each amount either in grams or in percentages (it depends on how you built your recipe).
- Contrary to The Sage, Soapcalc will give you a precise amount of liquid. In order to get the same average quantity, you have to choose "water as %age of oil" and fill in 30% (3). The automatic choice, 38%, will give you the maximum quantity on The Sage.
- You also have to choose the fat excess you want (automatically 5%) (4).
- Once the form is completed, click on "calculate" and "view recipe". You won't get any "brackets" here, only a precise quantity according to the choices you entered.
- Soapcalc
also has a "soap qualities" section, which I don't find very
useful or accurate. I'd rather use oils that I know have reliable values
and make my own tests (which again don't always fit with Soapcalc's
analysis).




Comments
Post a Comment